Avexitide for HI
Stage
Phase 3 ReadyDisease
Congenital HyperinsulinismPrevalence
1 in 25,000 to 50,000 births in USMechanism of Action
GLP-1 AntagonistRegulatory Designations
- Breakthrough Therapy in US
- Orphan in US and EU
- Rare Pediatric Disease in US
About Congenital Hyperinsulinism (HI)
HI is an ultra-rare, pediatric metabolic disorder resulting in persistent hypoglycemia in neonates and children
HI is characterized by fasting and protein-induced hypoglycemia and results in permanent brain damage with neurodevelopmental deficits in up to 50% of patients. Near-total pancreatectomy is often indicated and leads to life-long insulin-dependent diabetes (IDDM). Safe and effective therapies are urgently needed to prevent brain damage, IDDM, and death.
HI can result in permanent brain damage with neurodevelopmental deficits in up to 50% of patients
HI is congenital, rare pediatric disease with life-threatening manifestations of severe hyperinsulinemic hypoglycemia occurring primarily during the neonatal period, infancy, childhood, and adolescence. HI most often presents with severe hypoglycemia in the neonatal period requiring intensive care hospitalization, administration of high rates of intravenous glucose through central lines, continuous intravenous administration of glucagon, and, in many instances, surgical treatment by pancreatectomy during the neonatal period or during infancy, leading to lifelong insulin-dependent diabetes.
There are no FDA approved products labeled for HI.
Avexitide is an investigational glucagon-like peptide-1 receptor (GLP-1r) antagonist in development for treatment of HI
Avexitide binds to the GLP-1r on pancreatic beta cells and behaves as a GLP-1 antagonist and inverse agonist, reducing fasting and amino acid-induced cAMP accumulation and thereby decreasing calcium-stimulated insulin secretion.

- Dosed in multiple clinical studies at Children's Hospital of Philadelphia (CHOP)
- FDA Rare Pediatric Disease Designation
Avexitide: a targeted therapeutic approach
Loss-of-function mutations in the ATP-sensitive K+ (KATP) channel are responsible for the most common and severe form of HI (KATP HI), leading to increased GLP-1-mediated hyperinsulinemic hypoglycemia. This mutation leads to constitutive plasma membrane depolarization of the pancreatic beta cell resulting in elevated cytosolic Ca2+ and dysregulated insulin secretion. Avexitide binds to the GLP-1 receptor (GLP-1r) and lowers baseline cAMP levels, resulting in decreased insulin secretion despite elevated calcium levels. Similarly, by decreasing amino acid-stimulated cAMP accumulation, avexitide reduces insulin secretion.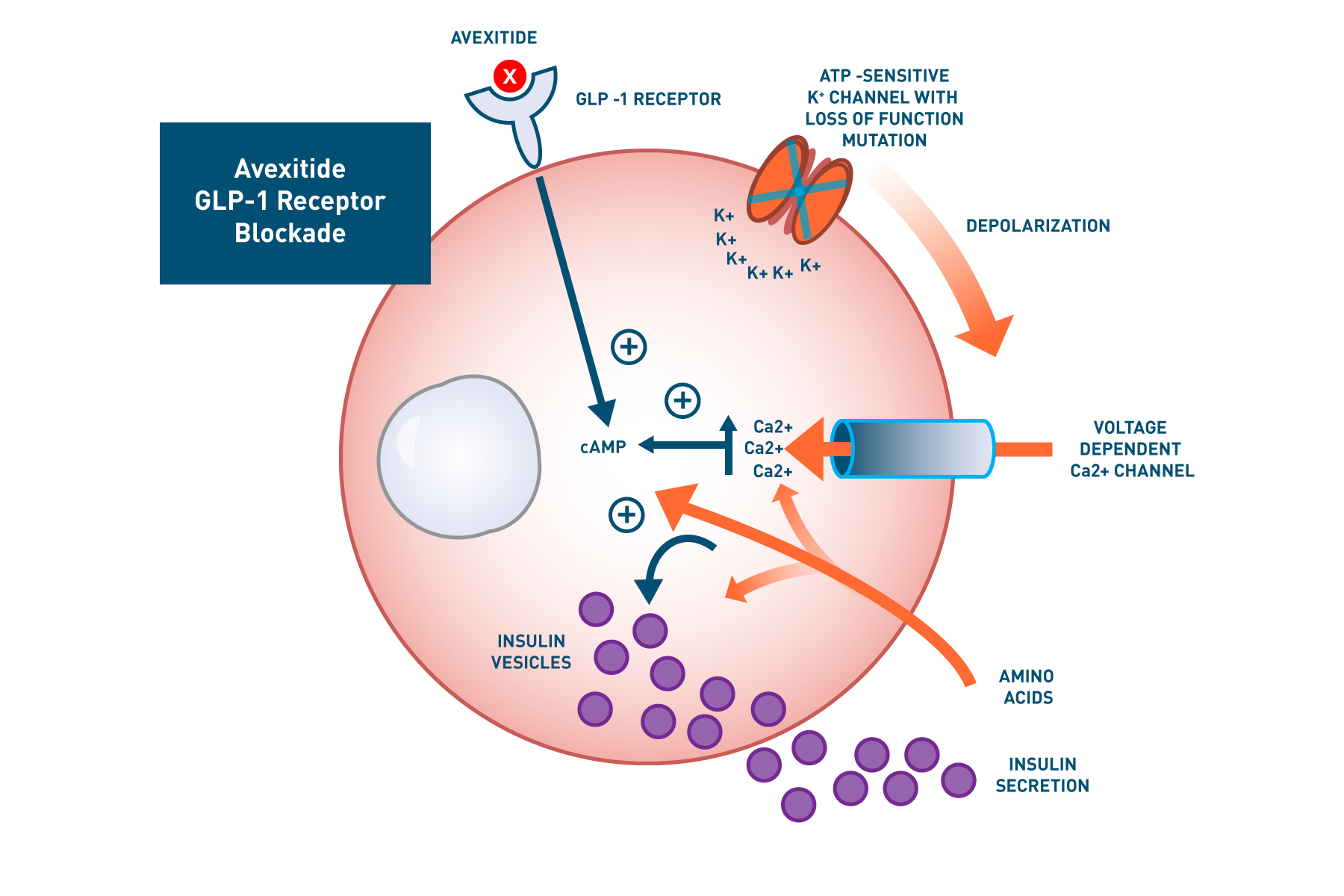

HI is an ultra-rare pediatric disease with Rare Pediatric Disease Designation in the US
The estimated incidence ranges from 1 in 2,500 live births in regions of high consanguinity to 1 in 50,000 live births. The estimated incidence in the United States is approximately 1: 28,000 resulting in an estimated number of new cases of HI each year in the United States of approximately 80-100. Because the severity of the Hypoglycemia in these children decreases with age, even if no pancreatectomy is performed in infancy, it is expected that children will require therapy with avexitide for a maximum of approximately 25 years, with the majority of treatment for serious disease manifestations required during the neonatal period, infancy, childhood, and adolescence. Thus, the total maximum number of cases eligible for treatment in any given year would be approximately 2,500.Avexitide in HI clinical studies
To date, a total of 39 patients with KATPHI enrolled in 3 clinical investigations at CHOP have received avexitide administered by continuous IV infusion: 10 adolescents and adults, 16 children, and 13 neonates. Data generated across all 3 clinical investigations suggest that treatment with avexitide may effectively reduce fasting and postprandial Hypoglycemia in patients with diazoxide unresponsive HI. Avexitide treatment was well tolerated, with no serious drug-related adverse events (AEs) reported. The most commonly reported adverse events to date were hypoglycemia and mild to moderate emesis, which did not lead to study discontinuation.| Study | # of Patients | Status |
|---|---|---|
| Adolescent and Adult Study | 10 | Completed Calabria AC et al. Diabetes. 2012;61(10):2585-2591 |
| Child Study | 16 | Completed |
| Neonate Study | 13 | Completed |
Avexitide in adolescents and adults study
Avexitide increased fasting glucose; decreased requirement for rescue
In a randomized, crossover, pilot study to evaluate the effect of a single, continuous infusion of avexitide or vehicle on glucose metabolism in 9 adolescents and adults with KATPHI, fasting blood glucose levels were statistically significantly higher during avexitide infusion compared with vehicle (105.4±23.2 vs 84.1±12.3 mg/dL, P<0.01). Eight of the patients had Hypoglycemia (blood glucose < 70 mg/dL) during vehicle infusion, of which 3 patients required an intravenous infusion of dextrose for symptomatic Hypoglycemia and blood glucose concentrations < 60 mg/dL. In contrast, no patients experienced Hypoglycemia during avexitide infusion. Overall, avexitide infusion reduced the insulin/glucose AUC as compared with vehicle (29.2 vs 43.6, P=0.053) with greater reductions in insulin/glucose AUC ratios observed with higher infusion rates (100 pmol/kg/min: 6.7 vs 8.9, P=0.11; 300 pmol/kg/min: 6.2 vs 10.3, P=0.016; and 500 pmol/kg/min: 6.0 vs 10.7, P=0.045).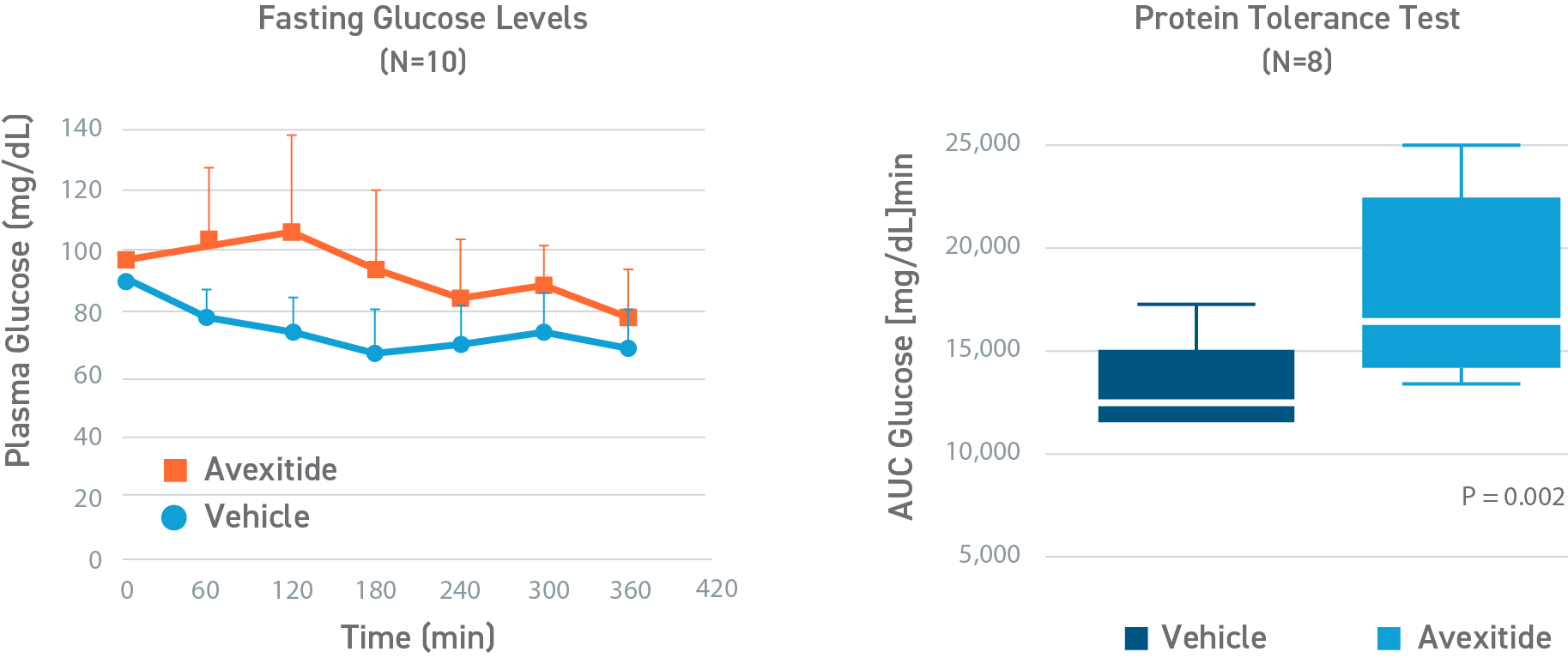



Avexitide in children with HI study
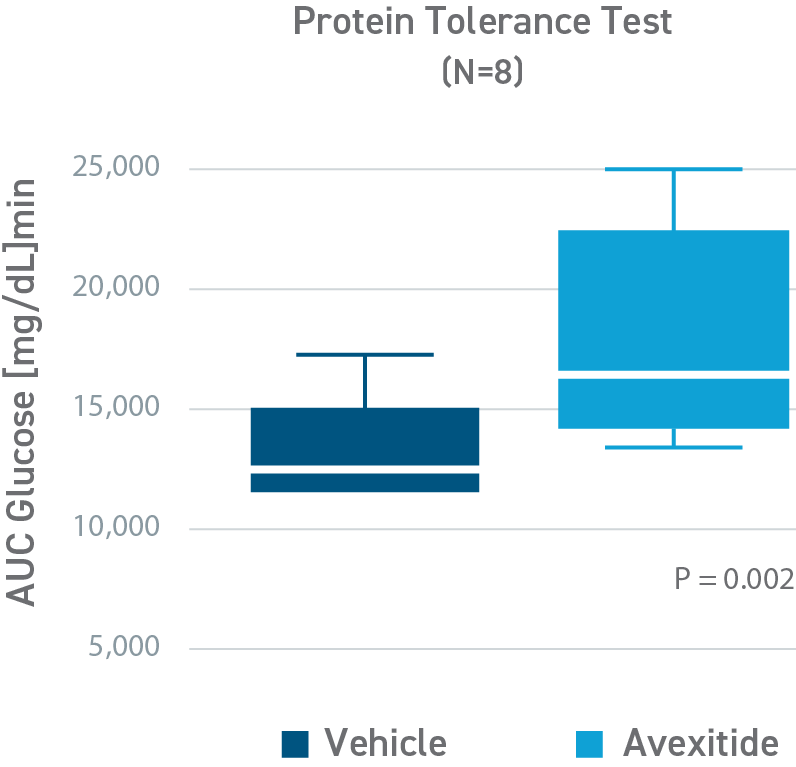
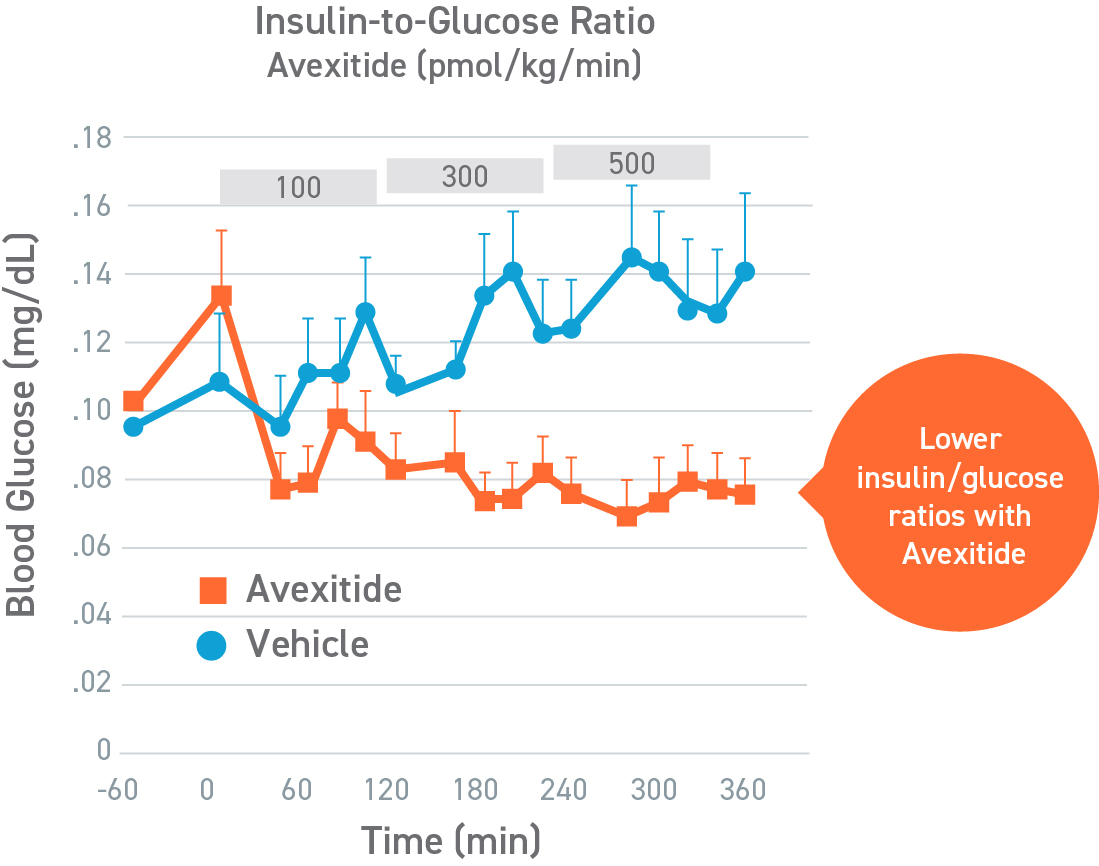
Avexitide raised fasting glucose and significantly reduced protein-induced hypoglycemia
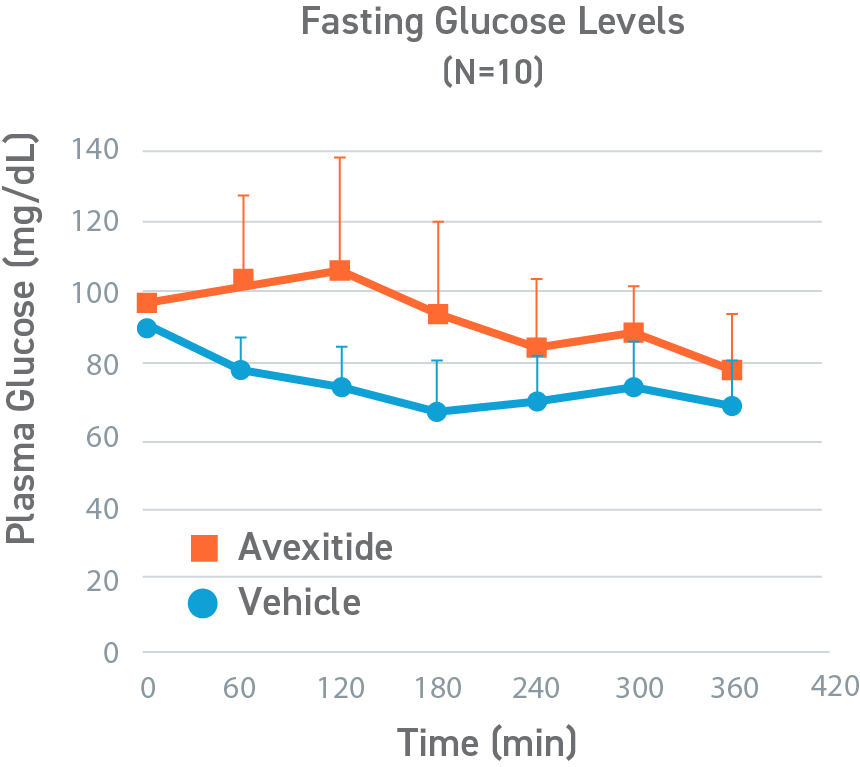
This was a placebo-controlled, randomized, crossover study designed to evaluate the effect of avexitide on fasting blood glucose levels (Days 1-2) and protein-induced Hypoglycemia (Days 3-4) in 16 children with HI due to mutations in the KATP channel. Patients aged 6 months to 18 years were randomly assigned to 1 of 2 treatment sequences: either an initial 6-hour IV infusion of avexitide on day 1 followed by a 6-hour IV infusion of vehicle on day 2 or vice versa. This study was conducted by CHOP (NCT00897676). Avexitide infusion resulted in higher fasting plasma glucose levels compared with vehicle, with greater separation between treatment and vehicle observed with the higher 500 pmol/kg/min infusion rate than the lower 100 pmol/kg/min infusion rate. Mean fasting AUC glucose concentrations were increased by 16%. Greater increases in fasting AUC glucose were observed with higher infusion rates: mean fasting AUC glucose was increased by 13%, 24%, and 7% for avexitide infusion rates of 500 pmol/kg/min, 300/500/300 pmol/kg/min, and 300/100/300 pmol/kg/min, respectively.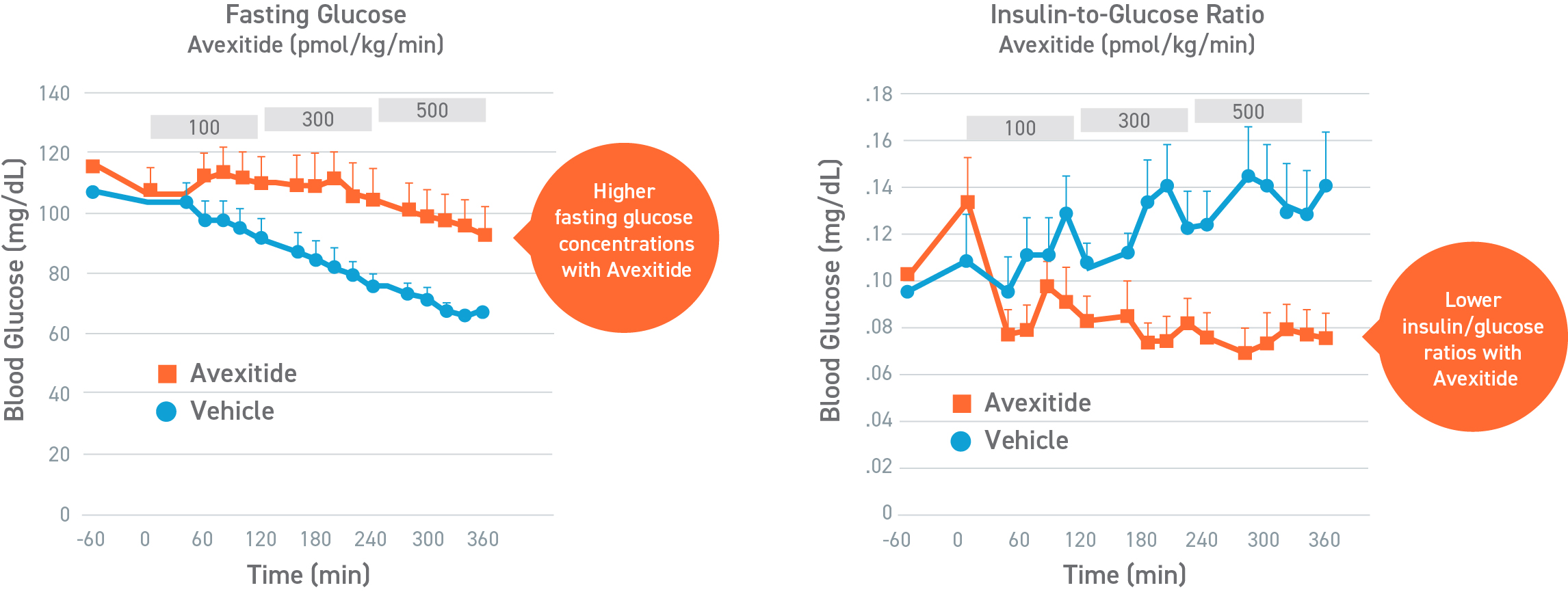


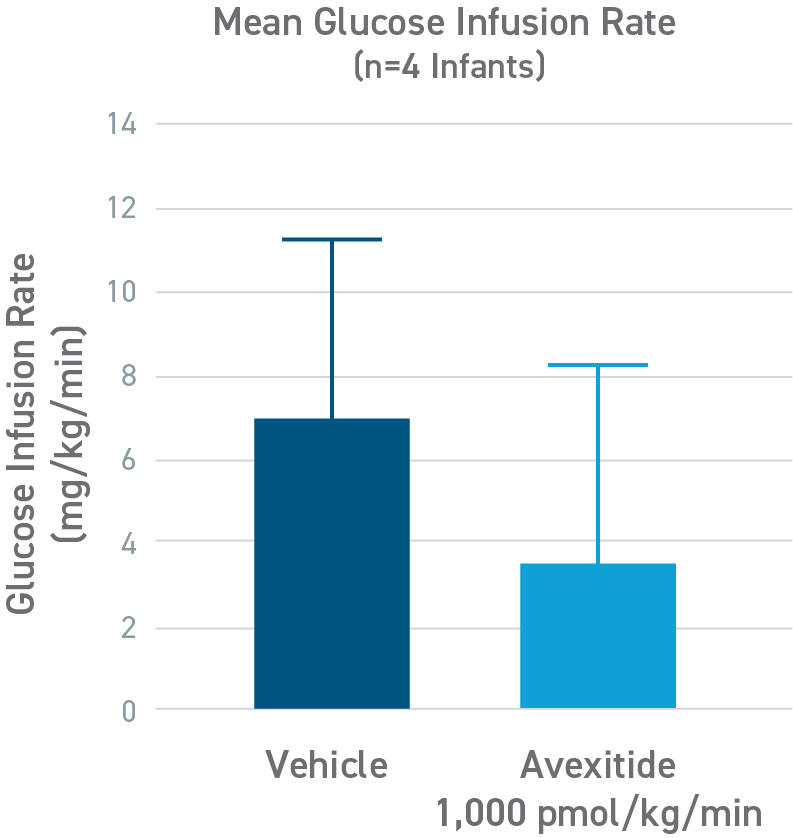
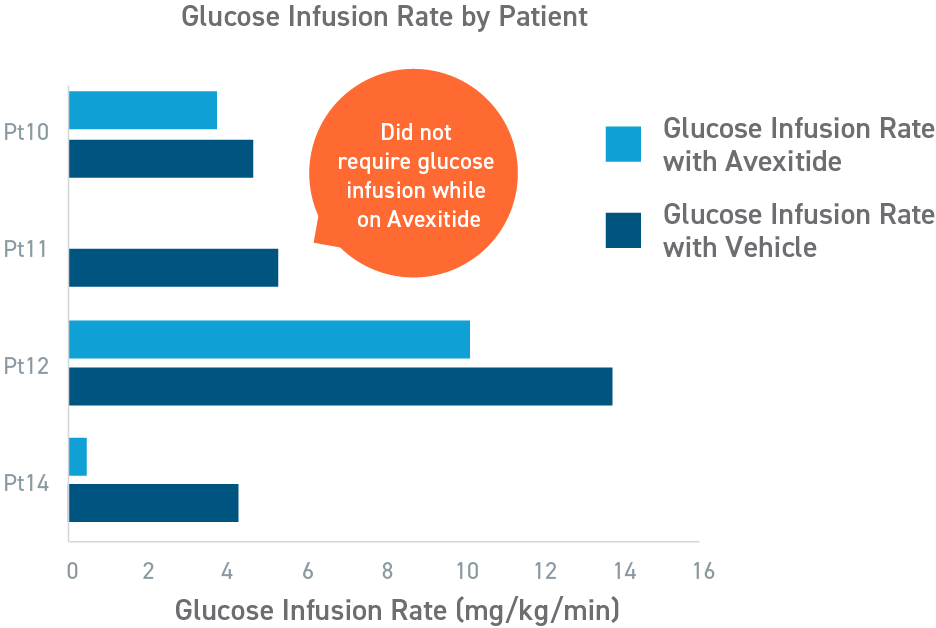
Avexitide in neonates and infants with HI study
Avexitide reduced mean glucose infusion rates by 60%
An open-label, randomized, crossover study to evaluate the effect of avexitide on glucose requirements to maintain euglycemia in 13 neonates and infants with diazoxide unresponsive KATP HI. The mean glucose infusion rate (GIR) during the last 2 hours of infusion was approximately 60% lower during avexitide infusion than during vehicle infusion. The GIR for each individual patient was reduced when receiving avexitide, and 1 patient did not require any glucose infusion during avexitide administration.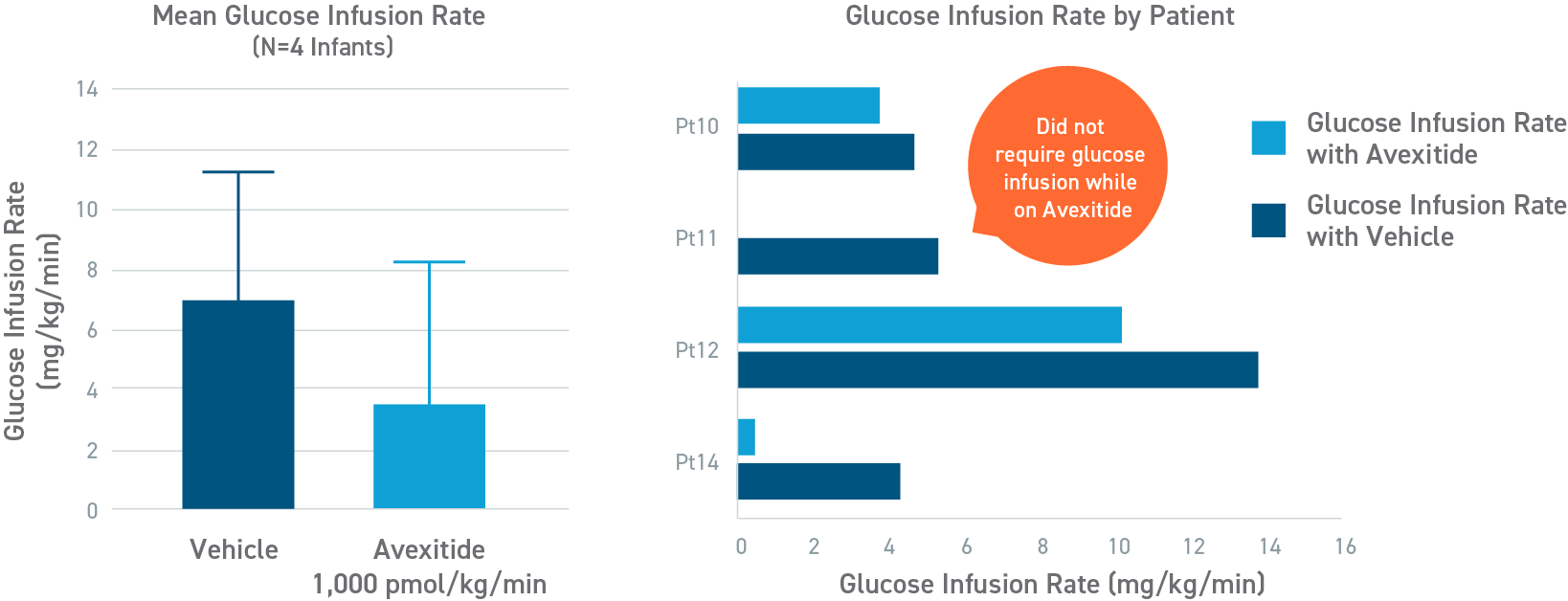



Avexitide Clinical Studies in HI
- Craig C and De Leon, D. "Pharmacokinetics and Pharmacodynamics of Avexitide [Exendin (9-39)] in Neonates and Infants with Congenital Hyperinsulinism." ADA 2021.
- Craig C et al. "Development of avexitide (exendin 9-39) for treatment of congenital hyperinsulinism." Poster presented at the CHI CHOP Congenital Hyperinsulinism Family Conference; September 6-8, 2019; Philadelphia, PA.
- Ng CM et al. "Population pharmacokinetics of exendin-(9-39) and clinical dose selection in patients with congenital hyperinsulinism." Br J Clin Pharmacol. 2018;84(3):520-532.
- Calabria AC et al. "Postprandial Hypoglycemia in Children after Gastric Surgery: Clinical Characterization and Pathophysiology." Horm Res Paediatr. 2016;85(2):140-146.
- Calabria AC et al. "GLP-1 receptor antagonist exendin-(9-39) elevates fasting blood glucose levels in congenital hyperinsulinism owing to inactivating mutations in the ATP-sensitive K+ channel." Diabetes. 2012;61(10):2585-2591.
Disease Overview, Etiology, Pathophysiology
- Li C et al. "Functional and metabolomic consequences of KATP channel inactivation in human islets." Diabetes. 2017;66(7):1901-1913.
- Lord K et al. "Clinical presentation and management of children with diffuse and focal hyperinsulinism: a review of 223 cases." J Clin Endocrinol Metab. 2013;98(11):E1786-E1789.
- De León DD et al. "Exendin-(9-39) corrects fasting hypoglycemia in SUR-1-/- mice by lowering cAMP in pancreatic beta-cells and inhibiting insulin secretion." J Biol Chem. 2008;283(38):25786-25793.
- De León DD, Stanley CA. "Mechanisms of Disease: advances in diagnosis and treatment of hyperinsulinism in neonates." Nat Clin Pract Endocrinol Metab. 2007;3(1):57-68.
